Poll on BSR Pharma activities in 2024
Baltic Sea Pharma Platform (BSR Pharma) aims to disseminate current knowledge and information on pharmaceuticals in the environment, focusing on effective ways to address this issue. BSR Pharma has been run by the Finnish Environment Institute for almost three years, continuing the groundbreaking work of the Swedish Environment Protection Agency.
Coordinating the BSR Pharma is a delight, yet it remains a continual challenge due to resource constraints. Thus, to ensure we use our resources optimally, we would like to leverage the expertise of the existing network to identify focus points for the BSR Pharma for the future. To develop the platform towards the needs in the field, we created a short (10‒15 min) poll. In this poll, we ask your interests and wishes towards the BSR Pharma.
One of the preliminary aims for 2024 is to organize a roundtable discussion between key stakeholders and sectoral agencies on the EU level. To help us elaborate on this plan, we have included in the questionnaire a couple of more specific questions about a roundtable discussion with relevant EU authorities, which BSR Pharma and PA Hazards are planning to arrange in 2024. The aim of the roundtable is to facilitate the knowledge sharing between authorities, focusing on the needs from the field.
Since only a limited number of people can join the roundtable discussions, we plan to arrange a preparatory workshop or webinar, where we wish to hear the latest developments in the field that we can share to the EU authorities as well as your needs and wishes for the roundtable discussion topics.
Additionally, we would like to hear what are the best ways to share the outcomes of the roundtable meeting to a wider audience.
Please, take this short poll and help us plan the activities for this year! We appreciate all responses submitted by Friday, February 16th. https://link.webropolsurveys.com/S/A60817AF9D27DAD3
Wishing you health and peace on the new journey around the sun,
The BSR Pharma team
Lauri Äystö & Noora Perkola
PharmaSea: Which are the environmentally hazardous pharmaceuticals in the Baltic Sea?
Author: Daniel Malnes, Project Researcher, PharmaSea, daniel.malnes(at)helcom.fi
Pharmaceuticals have recently been detected in the Baltic Sea environment. As several countries share the Baltic Sea, cooperation is needed to answer important questions: What is the status of current knowledge, and what needs further investigations?
The initiation of a new HELCOM project
In a new project PharmaSea, HELCOM in collaboration with Contracting Parties will answer important questions related to the Baltic Sea environment. Through the collection of environmental occurrence data, sales data, and evidence of environmentally hazardous properties in the Baltic Sea, the work aims to summarise the findings at the end of 2024. Some preliminary insights are however already available.

Figure 1. Countries which have contributed to HELCOM’s occurrence data call for pharmaceuticals.
Which data is available?
Several countries around the Baltic Sea have provided environmental occurrence data for pharmaceuticals (Figure 1). This data spans several important environmental matrices: different types of waters (wastewater, surface waters collected from rivers, lakes and coasts, as well as marine waters), sediments, and biota. These matrices will provide important information about national usage (wastewaters), environmental fate processes (rivers, lakes, coastal waters and sediments), and the pharmaceuticals ultimately reaching the Baltic Sea (marine waters and marine biota).
An estimated 250 pharmaceuticals, metabolites and transformation products have been monitored across all monitored environmental matrices. The most monitored pharmaceuticals (Figure 2) are typically the pharmaceuticals on the European Union’s Water Framework Directive Watch list and the ones commonly detected in environmental matrices. Dependent on their occurrence in the Baltic Sea environment, environmentally hazardous properties will be evaluated. The evaluation could include (i) environmental persistence, (ii) comparison against concentrations which are chronically toxic for aquatic fauna, (iii) bioaccumulative properties of the contaminants, and potentially more.
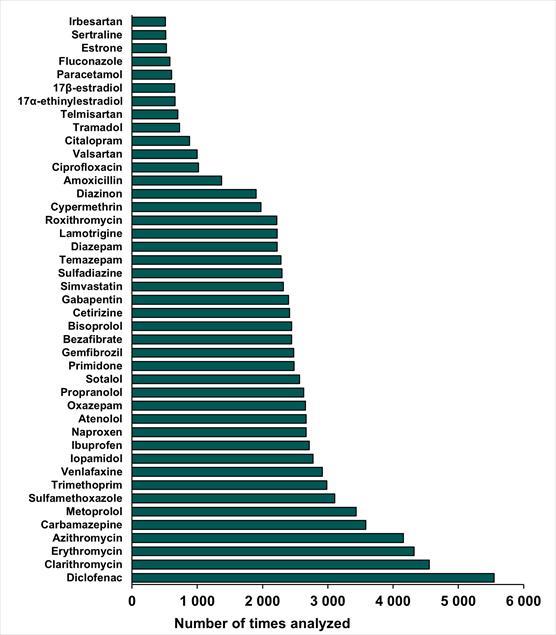
Figure 2. Pharmaceuticals which have been monitored the most across environmental matrices. The graph only displays pharmaceuticals measured more than 500 times.
The project PharmaSea further aims to collect sales data during 2024 to identify data gaps in, and potentially complement, the environmental monitoring data in the Baltic Sea region. Pharmaceutical usage can differ from country to country, which is why the project aims to identify the priority pharmaceuticals for the Baltic Sea environment. With this approach the ambition is to counter feedback loops where the same contaminants are monitored continuously, potentially missing other hazardous contaminants. The results of the work will be summarised in a report by the end of 2024.
Interreg BSR Project APRIORA – prioritising pharmaceutical emission sources and receiving rivers by an improved risk-assessment approach
Authors:
Jens Tränckner, University of Rostock, jens.traenckner(at)uni-rostock.de
Alena Seidenfaden, University of Rostock, alena.seidenfaden(at)uni-rostock.de
In November, the new Interreg Baltic Sea Region (BSR) project APRIORA was kicked off in Rostock, Germany. Nine partners in the BSR unite to tackle a huge need: Transfer knowledge and tools regarding pharmaceuticals in the environment to authorities in charge of prioritising emission sources. Why is this a specific challenge?
Broad knowledge on pharmaceuticals in the environment
As stated in HELCOM reports, it is well known that emitted active pharmaceutical ingredients (API) from point sources are posing a long-term risk to the BSR, both in the river catchments and the Baltic Sea. The highest amount of human pharmaceuticals is discharged by domestic wastewater treatment plants (WWTP).
Already, many successful forerunner projects, focussed on pharmaceuticals in the environment, have studied, monitored, modelled and produced a lot of data and created a broad knowledge on the subject. The projects “CWPharma” and “MORPHEUS” worked with project specific foci on monitoring, modelling and risk assessment approaches, while the projects “Less is More” and “BONUS Cleanwater” developed pilots of technical mitigation measures. Most participating organisations excel in research or transnational political procedures. While authorities overseeing river (sub-) catchments have been involved in previous projects, many lack the full capacity to perform detailed API risk assessments independently. So far, there does not exist a widely established risk assessment framework for APIs in WWTP recipients within the BSR catchment.
Upcoming challenge for authorities in charge – the new UWWTD
Currently, the new revision of the European Urban Wastewater Treatment Directive (UWWTD) strongly fosters the implementation of targeted mitigation measures to reduce the emission of micropollutants, including APIs. (Read more about the UWWTD in BSR pharma newsletter I/2023). According to the proposal published in autumn 2022, all larger WWTPs (exceeding 100,000 PE (population equivalent)) shall be equipped with advanced treatment for organic micropollutants. Further, a risk-based prioritisation of advanced treatment will be required from all WWTPs between 10,000 and 100,000 PE.
These smaller WWTPs are highly characteristic for the Baltic Sea Region. In the BSR there are more than 1,000 WWTPs in the range of 10,000 to 100,000 PE, and more than 80% of them are located inland and in rural areas. There is one thing missing in the regulation altogether: the fact that there exist about ten times more WWTPs under 10,000 PE. These WWTPs often have low treatment. Furthermore, they often discharge in small and highly vulnerable surface waters where even small local emissions may have relevant impact on water quality.
It is not affordable to equip all WWTPs with advanced treatment, but a consistent mitigation strategy requires considering all emission point sources, the respective receiving water systems as well as various risks, following the WHO “one health” approach. However, monitoring of APIs and flow is limited and it provides mostly only local, transient insights. The corresponding assessment of environmental and human-related risks is still pending, too. All in all, the authorities in charge will face a huge challenge implementing the ambitious objectives of the new UWWTD.
What is APRIORA and its strategy?
Project partners from Sweden, Poland, Latvia, Finland and Germany are now combining their competencies in the APRIORA consortium (see Table 1). The nine partners have already worked together in previous forerunner API projects. So far, 19 associated organisations support the consortium. Most of the associated organisations are authorities in charge, but there are also wastewater organisations and WWTP operators active in the pilot areas in the five BSR countries involved.
Table 1. Project partners
|
Country
|
Partner
|
| Germany |
- University of Rostock (URO)
- German Environment Agency (UBA)
|
| Finland |
- Finnish Environment Institute (Syke)
- Centre for Economic Development, Transport and the Environment in South Ostrobotnia (ELY)
- Finnish Medicines Agency (FIMEA)
|
| Latvia |
- Latvian Institute of Aquatic Ecology (LIAE)
- Latvian Environment, Geology and Meteorology Centre (LEGMC)
|
| Poland |
- Gdansk University of Technology (GUT/Gdansk Tech)
|
| Sweden |
- Kristianstad University (HKR)
|
The full title of the project summarises its main objective: “Improved risk assessment for strategic water management to reduce micro-pollutant emissions in the Baltic Sea Region”. This shall be achieved by a spatial high resolution estimation of pharmaceutical emissions and API concentrations in the ambient water system, combined by an integrated risk assessment approach. The development and transfer of such approach can only be successful when accompanied by good communication with the target groups. For the project to fully integrate the needs of the target groups and for the target groups to adapt the outcomes, the project reaches out to the stakeholders and intended applicants at an early stage of the implementation.
General Concept – more details on APRIORA approach
Fortunately, APRIORA does not start from scratch but can build on the above-mentioned forerunner projects in the Baltic Sea Region and research worldwide. However, the APRIORA approach is unique in its clear focus on a holistic concept for impact assessment and prioritisation of emission sources and mitigation measures.
The basic idea is to estimate individual WWTP discharges and related API concentrations in receiving river sections by combining statistical consumption data with WWTP, hydrological data and a simplified calculation of transport at the surface water system. These will feed the subsequent risk assessment, categorized in three different criteria: i) impact on aquatic environment, ii) formation of antimicrobial resistances, iii) hazards to downstream water usage and human health. The approach shall also provide functions to pre-assess the efficacy of technical mitigation measures at individual WWTP. To ease the application of the developed framework by the authorities and operators, APRIORA will provide supporting digital tools implemented in daily-work environment, namely GIS.
Calibration and uncertainty assessment of the simplified emission and transport model is mandatory for a reliable application. This requires targeted monitoring concepts with a condensed list of characteristic and well detectable parameters and wisely chosen locations for sampling and flow measurement. Therefore, designing according monitoring concepts is one crucial part at the beginning of the project. The whole concept depends strongly on the efficient management and processing of geo-data.
The APRIORA´s concept is challenging not only for its developers but also for the target groups. Therefore, an initial capacity building is mandatory. Accordingly, the APRIORA team will establish a digital learning platform with background information and step-by-step tutorials, supplemented by hands-on trainings for different topics. Already at the very beginning, authorities in charge are interviewed to fit the training concepts to their current needs which are expected to be at different levels in each BSR country.
Project Timeline & Call for interested target groups in BSR
The different modules of the framework will be developed in parallel working groups, coordinated by a compact steering group. During the first project year, the concepts are elaborated and accompanied with solid background documentations and pilots are prepared. In project year two, the developed framework and the supporting GIS-tools shall be piloted in five exemplary river basins, one in each country. The third project year will be a year of intense capacity building for the project partners and associated organizations as the knowledge obtained will be rolled-out to the Baltic Sea Region.
The APRIORA outcomes will improve knowledge of cost-efficient mitigation measures. The outcomes are likely to benefit both the authorities in charge and WWTP operators. The APRIORA approach could prove applicable not only to APIs but also to other hazardous compounds and mitigation techniques at wastewater treatment. The APRIORA project also targets in cooperation and fruitful exchange with other Interreg research projects, such as EMPEREST and AdVIQWater.
APRIORA questionnaire awaits your response
Currently, the project reaches out to target groups with an online questionnaire on API monitoring practices in BSR (Link to the questionnaire). Expert knowledge at national, regional, and local levels will be collected and analysed to identify best practices. This approach ensures that target groups are involved from the outset to define the initial situation and needs. Anyone working in a related field is welcome to contribute actively! Please submit responses to the survey by February 9, 2024.
If this article has gained your interest either as authority in charge, WWTP operator, research project partner or in any other role, the APRIORA team is happy to get in contact with you and your organisation.
Contact APRIORA:
APRIORA Project manager, Communication manager:
Alena Seidenfaden,
University of Rostock,
alena.seidenfaden(at)uni-rostock.de
More information on APRIORA website: https://interreg-baltic.eu/project/apriora/
References
European Commission 2022a. Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL concerning urban wastewater treatment (recast). Brussels, 26.10.2022, COM(2022) 541 final. 2022/0345 (COD)
New PharGTrans project examines whether changes in EU chemicals policy and legislation that support the green transformation affect the pharmaceutical sector
Author: Mirella Miettinen, University of Eastern Finland, mirella.miettinen(at)uef.fi
‘Green transformation’ is an ambiguous concept, but the general idea is to move towards sustainability to combat global environmental change caused by human activities. The European Union (EU) has a large pharmaceutical market and a competitive pharmaceutical industry. However, the shift to a greener design and production of pharmaceuticals will require transformation by many pharmaceutical actors in the EU.

© Gerd Altmann from Pixabay.
The need for empirical studies
There are few empirical studies on how different actors operationalise transformation in practice. In addition, the cross-cutting effects of policies and legislation, such as the European Green Deal and the resulting changes to EU chemicals legislation, have not yet received much attention in the pharmaceutical sector.
The four-year PharGTrans project, funded by the Research Council of Finland, explores how changes in EU chemicals policy and legislation may affect green transformations in the pharmaceutical sector in the EU. The project will conduct an interview study among various stakeholders in the pharmaceutical sector to gather new qualitative data on the perceptions and drivers of green transformation. The stakeholder groups interviewed will cover researchers, industry, interest groups, public authorities, and political decision-makers.
Four universities are involved
The project is led by Senior Researcher and Academy Research Fellow, Docent Mirella Miettinen at the University of Eastern Finland Law School. The project partners include the Universities of Eastern Finland, Helsinki, Stockholm and Aarhus representing law, pharmacy and environmental sciences. Pharma Industry Finland (PIF) and the European Federation of Pharmaceutical Industries and Associations (EFPIA) are also collaborators in the project.
The research is grounded on an interdisciplinary collaboration between researchers in environmental law, pharmacy, and environmental science. One of the project goals is to establish a network of researchers that brings together expertise in law, pharmacy and environmental sciences and provides training for young researchers.
The project is a continuation of the SUDDEN project
PharGTrans is a continuation of the SUDDEN project, which was funded by the Strategic Research Council at the Research Council of Finland in 2018–2023. SUDDEN focused on the environmental impacts associated with the life cycle of pharmaceuticals, with a second objective of enhancing the sustainability of the pharmaceutical sector.
The legal work carried out in SUDDEN examined the relevant regulatory frameworks at international, EU and national level. One of the overarching findings was that the pharmaceutical industry is under increasing regulatory pressure to address sustainability and environmental concerns, particularly at EU level.
Results help to support transformation paths both in practice and in policy making
PharGTrans’s analysis of policy and legal documents and interviews focus in particular on the design and manufacturing stages of the pharmaceutical life cycle. The empirical findings will be combined with the scholarly literature on green transformation in order to synthesise insights on the depth and triggers of green transformation pathways in the pharmaceutical sector and to provide recommendations on how to support this transformation in practice and in policy making.

Do you have something you would like to share to the BSR PHARMA?
Tips on contents for future newsletters are welcomed at BalticPharma(at)syke.fi