7.23.1 Introduction
Toxification of the environment is related to the introduction and dispersion of chemicals to such an extent that adverse effects may be invoked in plants, animals or man. The number of chemicals introduced into the environment has grown exponentially over the past century. Of the approximately 10-million substances known at the present day, about 100 000 have toxic properties which make them a potential subject of environmental policy and control. Due to toxicant diversity it is virtually impossible to monitor all toxic compounds and their effects individually. The groups of toxicants currently most important for the assessment of large-scale air pollution are the heavy metals (HM) and the persistent organic pollutants (POPs).
The intrinsic toxicity of heavy metals (zinc, cadmium, copper, mercury, lead, chromium, nickel and arsenic) is documented in the international literature. However, the bioavailability, or the actual exposure concentration experienced by biota is very much related to the local situation with respect to masking by complexation. This in turn is related to e.g. acidification, redox-potential and soil type (CEC and organic contents). For heavy metals, the organisms in chronically exposed populations generally possess adapted excretion mechanisms, leading to reduced exposure levels and thus reduced effects.
The situation is much more complex regarding the toxicity of the organic compounds. Due to the high structural diversity, the intrinsic toxicity of many organic contaminants is unknown. However, the level of volatility and persistence required for organic contaminants to be airborne for a longer period of time, puts a limit on the types of compounds of interest. The most important groups of airborne organics are the chlorinated organic substances (PCBs, pesticides, dioxines, etc.) and the polycyclic aromates (PCA), which have well documented toxicities.
The ecological effects possibly occurring as a consequence of the excessive presence of toxicants are very diverse. This is because the effects can be exerted along different major pathways. Some effects are directly related to the uptake of toxicants, which then can interfere with physiological processes according to their mode of action. The uptake of toxicants can either be direct from the environment or through the food chain.
- Direct uptake from the environment is mainly producing effects in primary producers and small animals living in close contact with water. Water soluble toxicants are directly taken up from surface, rain or soil water, while gaseous compounds are absorbed by the leaves of plants. The resulting toxicant concentration inside the exposed organism, depends on the uptake rate and the rate of excretion, the rate of toxicant conversion (degradation) or the rate of dilution by growth. Given adequate time with constant exposure, the toxicant concentration in the organism will reach an equilibrium, where the internal concentration of the toxicant is generally considerably higher than the environmental concentration. This process is called bioconcentration. For organic substances the bioconcentration factor is mainly governed by the lipophilic properties of the toxicant and the fat content of the exposed organism. Bioconcentration of heavy metals may be controlled by the ability of the exposed organism to reduce inherent metal toxicity by forming comparatively harmless complexes (e.g. with metallothioneine). The resulting internal equilibrium concentration may be below or above the threshold concentration for effects.
- Exposure through the food chain is of increasing importance for terrestrial herbivores, detrivores and carnivores, respectively. Due to a low (approx. 10%) metabolic conversion factor, these animals need to ingest relatively large portions of food to fulfill their energy requirements. This food may have comparatively high concentrations of bioconcentrated toxicants, which may lead to a high rate of toxicant uptake. In general, the excretion or elimination rate of toxicants is observed to be lower in terrestrial air breathing animals than in aquatic organisms with respiratory gas exchange with the water (skin/gill respiration). The resulting higher body burden of toxicants in terrestrial animals makes them more vulnerable to toxicants which are passed on through the food chain. For truly aquatic species, the comparatively high excretion rate causes the difference in body burden between direct uptake and food chain transfer to be less pronounced. The process resulting in a higher body burden of toxicants through the food chain is called biomagnification. The overall effect of a higher toxicant concentration in the tissues of exposed organisms then in their environment, irrespective of the exposure pathway, is called bioaccumulation.
- Excluding the effects related to the uptake of toxicants, organisms can also suffer indirect effects originating from toxicity related changes in community structure. For a particular species, these effects can be negative in case of reduced availability of food organisms, and positive in case of reduction in the abundance of competitive species.
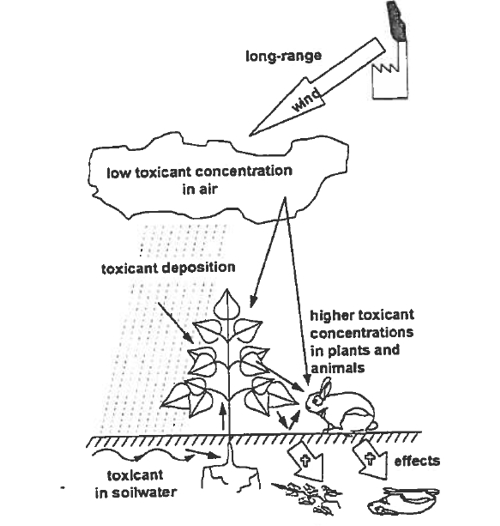
From the first part of this introduction on toxicity assessment, it will be clear that an identification of options for toxicity monitoring requires the chain of causes and effects to be studied in detail as depicted in figure 7.23.1.
|
|
|
|
|
Figure 7.23.1. Simplified cause-effect chain.
|
For proper evaluation of toxicity, it is essential that the determination of concentrations and loads of a wide variety of toxicants in air, deposition, soil, soilwater, ground water and surface water is covered under the appropriate subprogrammes. However, for some of the contaminants to be expected, the concentrations in these media may be well below the chemical analytical detection limit. It is therefore indispensable to assess ecotoxicological effects occurring in the local biological community as the consequence of prime concern, together with the toxicant concentration inside exposed organisms as the immediate causative factor.
In the chain of causality for ecotoxicity, the effects on biological systems are primarily decisive as to the seriousness of the impact. Especially the occurrence of indirect effects makes it very difficult to conclude toxicological effects by analysing the results of inventories on community structure. In other words: toxicological effects may be there, but can not be distinguished from effects on community structure resulting from other disturbing factors. Analysing shifts in community composition in concert with observed body toxicant burden in target organisms may reveal toxicity induced community responses. A regular estimate on community composition of plants and birds is covered by the separate subprogrammes VG and BB.
Ecotoxicity can also be estimated by controlled experimental exposure of test organisms to serial dilutions of environmental samples. Two types of laboratory bioassays can be distinguished:
- The easiest, cheapest and least sensitive methods are targeted towards the determination of the external concentration at which acute effects are invoked in the exposed test organisms. Acute effects are generally defined as mortality occurring within 1 to 4 days of exposure. However, in aquatic ecotoxicology there is a tendency for the development of short-term tests solely relying on subtle physiological or cytological changes, which may even occur in-vitro (biomarkers; cell cultures, isolated enzymes, etc.). Several validated acute test systems exist for exposure through the aquatic and soil environment. Ecotoxicological tests for air exposure have not (yet) been developed. Especially in terrestrial organisms, the short exposure duration may prevent the partitioning process to reach equilibrium with the internal toxicant concentration experienced by the test organisms. Furthermore, these tests deliberately exclude effects that may occur through the food chain process of biomagnification. For the ICP IM monitoring programme, mainly executed at sites which are relatively undisturbed by local sources of pollution, it is therefore very unlikely that acute toxicity testing will demonstrate any effects, even when the environmental sample is tested without dilution.
- Prolonged exposure experiments are capable of detecting sub-lethal effects (retarded growth, reduced reproduction, etc.) and will include effects linked with accumulation and food chain magnification. These types of toxicity tests are generally much more sensitive, and may be able to detect the toxicity associated with long-range dispersion of heavy metals and POPs as air pollutants. For the aquatic environment, some standardised and validated semi-chronic tests are available, with a test duration of 4 to approximately 30 days, depending on the type of test organism (algae, daphnia, fish). A limited number of prolonged tests on soil or soil water ecotoxicology are still in the process of being standardised and validated, while tests for air exposure are non-existent. However, the execution of all prolonged exposure tests is extremely costly, requiring specialised facilities and highly trained personnel, which renders widespread application in a monitoring network virtually impossible.
When, due to natural variability, the relationship between community responses observed in the field and ecotoxicity can only be assessed with great difficulty and uncertainty and the execution of environmental bioassays for the detection of contamination introduced by long-range air pollution is considered to be unfeasible, the only solution to the problem of toxicity assessment is to analyse for bioaccumulation of selected toxicants in target organisms. Because the observed internal concentrations of toxicants only reflect toxicity in a relative manner - as long as models for predicting community or population effects from bioaccumulated toxicant concentration are not yet fully developed - the target species should be common organisms with a widespread occurrence. Furthermore, the target species should be relatively insensitive towards the bioaccumulated toxicant level, in order to produce an wide range indication of potential toxicity The determination of bioaccumulated toxicants has the additional advantage over the determination of toxicant levels in air, soil or water, that the target species will integrate the contamination over time, so that monitoring frequency can be comparatively low.
In the subprogrammes on foliage (FC) and litterfall chemistry (LF) as well as in the subprogramme on metal chemistry of mosses (MC), some determinations are made on the internal concentrations of mainly heavy metals. Chemical analysis of a larger variety of toxicants in a larger variety of species or specific parts of species will greatly enhance possibilities for ecotoxicological risk assessment. Many additional target species with established bioaccumulative capacity for specific groups of contaminants are described in literature:
- Duck weed (Lemna sp.) is known for its accumulation of heavy metals over a wide concentration range (e.g. Jenner et al., 1993)
- Many species of lichens are used for analysis on the accumulation of heavy metals, polycyclic aromates and organo-chlorines (Calamari et al., 1991)
- The concentration of volatile organochlorine compounds in bark, leaves and needles of selected tree species is widely used for assessing the level of air contamination (Simonich et al, 1995ab; Thompson et al., 1995)
- Fish species with a high fat content (e.g. Eel; Anguilla sp.) are good indicators for the bioaccumulation of persistent organics (RIZA, 1996)
- The tissue of both fresh water and marine mussels (e.g. Dreissena sp. and Mytilus sp.) is globally used to assess the level of mercury pollution (Musselwatch Programme; NAS, 1980), cadmium pollution and PCB pollution (e.g. Mersch et al., 1992)
- Lower soil organisms like the earthworms (Lumbriculidae) and the more commonly occurring species of the threadworms (Enchytraeidae) as well as wood lice (Isopoda) are capable of accumulating heavy metals (Martin and Coughtrey, 1982; Hopkin, 1989) and organic pollutants (Callahan et al., 1991)
In general it can be stated that the accumulation of toxicants by biota is subject to much variability. Between remote populations of a single species, the variation is mainly introduced by adaptation differences in the efficiency of excretion and metabolic degradation. Within a local population the differences in accumulation are mainly governed by the age of individuals and their nutritional status. It is therefore essential to analyse composite samples of a relatively large number of randomly picked individuals of approximately the same age. For heavy metals the determinants should be normalised to unit dry weight, while the accumulation of organic pollutants is best expressed per unit lipid content.
7.23.2 Methods
In analysing and reporting bioaccumulated contaminants, there is a lot of freedom in procedures which can be executed to obtain correct and interpretable results. This chapter only gives a very simplified outline of possible procedures for both heavy metal and organic contaminants All procedures consist of the following steps required:
- Composite sampling
- Sorting
- Cleaning
- Composite Sub-sampling for replicates
- Transport storage
- Prolonged storage
- Sample pre-treatment
- Sample clean-up
- Analysis
- Data preparation
- Data reporting
7.23.2.1 Field methods
Adequate numbers of the required material are collected in the field. Immediately after collection, the sampled animals or parts of plants are hand-sorted to uniform age and composture. If necessary, the material is rinsed clean of debris with distilled water. Triplicate composite samples containing sufficient material (10-20 grams) are composed to be analysed independently. The unpreserved composite samples are immediately stored in PE containers or suitable plastic bags, which should be kept cool (+4°C) and in the dark for a short period of time until they can be transferred to a freezer (-20°C) in the laboratory.
7.23.2.2 Laboratory methods
If samples require pre-treatment, as for instance the removal of shells from mussels, or the dissection of target organs from larger animals, this has to be performed prior to freezing. The samples can be stored under refrigeration for a prolonged period of time. Upon analysis, the samples are freeze-dried and subsequently ground with an agate mortar to obtain a dry powder.
Trace metal analysis
Arsenic, cadmium, chromium, copper, lead, nickel, zinc
An adequate and metered amount of the dry powdered sample is mineralised by a suitable wet acidic-oxidative destruction procedure. The resulting liquid sample is topped to known volume with distilled water and filtered. The filtrate can be kept in PE bottles prior to AAS or ICP analysis.
Mercury
Mercury requires leaching of samples with acid inclosed vessels followed by cold vapour AAS.
Analysis of organic residues
A known weight of dry powdered sample is subject to extraction of the lipophilic fraction. The first extraction is performed by prolonged (16 hrs) shaking with a hexane/acetone mixture (3 : 1). The extraction is repeated three times with decreasing extraction periods and decreasing solvent volume. In order to determine the lipid contents, the solvents are evaporated to dryness and the residue is weighed. The extract is then dissolved in a small amount (ml)of hexane and purified by addition of some 95% sulphuric acid. After mixing and separation of the two resulting phases by centrifugation, the cleaned-up hexane solution is analysed by appropriate LC, GC or GC-MS methods.
7.23.3 Quality assurance/Quality control
With all types of analysis standard addition techniques are used to account for matrix interactions. Procedural blanks are run to assess contamination possibly occurring during the different stages of sample preparation. Various chemical-analytical supply houses offer a variety of certified standards containing biological tissues with known amounts of organic and inorganic environmental pollutants. This type of standards can and should be used to validate sample pre-treatment and analysis.
7.23.4 Data pre-treatment
The data should be normalised to unit dry weight for the heavy metal content of organisms, and to unit lipid content for organics. The arithmetic average, geometric average, standard deviation, and number of replicate analytical results for the same date-place-species-compound combination should be calculated and reported separately.
7.23.5 Data reporting
Variables
All measured individual toxicants or summary variables (e.g. AOX) should be reported.
|
COL
|
CONTENTS
|
EXPLANATION
|
EXAMPLE
|
ABBREVIATION OF
|
|
1-2
|
Subprogramme
|
Obvious
|
TA
|
Toxicity Assessment
|
|
3-6
|
Area
|
Obvious
|
NL01
|
Netherlands 01
|
|
7-8
|
Institute
|
Obvious
|
RV
|
RIVM
|
|
36137
|
Station
|
Obvious
|
6
|
Fen Kliplo
|
|
36179
|
Medium
|
Analyzed species
|
ANGU ANG
|
Anguilla anguilla
|
|
21-22
|
Medium list
|
Obvious
|
F1
|
Fish
|
|
23-26
|
Level
|
Not Applicable
|
|
|
|
27-32
|
Year+month
|
YYYYMM
|
199711
|
November 1997
|
|
33-34
|
day
|
DD
|
5
|
day of the year and month above
|
|
35-37
|
Spatial pool
|
Number of replicates
|
3
|
3 replicates
|
|
38-45
|
Substance
|
Chemical agent
|
PCB123
|
PCB 123
|
|
46-47
|
Substance list
|
Obvious
|
DB
|
DB
|
|
48-50
|
Pretreatment
|
Code for pretreatment method
|
|
|
|
51-53
|
Determination
|
Code for determination
|
|
|
|
54-60
|
Value
|
Obvious
|
1.25
|
1.25
|
|
61-68
|
Units
|
Obvious
|
mg/kglc
|
mg per kg lipid content
|
|
69-69
|
Quality flag
|
Not Applicable
|
|
|
|
70-71
|
Status flag
|
Way of calculation
|
G
|
Geometric average
|
|
|
or
|
|
D
|
Standard deviation
|
|
|
or
|
|
X
|
Arithmetic average
|
|
72-72
|
Additional field
|
Not Applicable
|
|
|
Additional status flags D (standard deviation) and G (geometric average) used here.
7.23.6 References
Calamari D, E Bacci, S Pocardi, M Morosini and M Vighi. M. Environ. Sci. Technol., 1991, 35: 1489-1495.
Callahan CA, CA Menzie, DE Burmaster, DC Wilborn and T Ernst. Environm. Contam. and Toxicol., 1991, 10: 817-826.
Jenner HA and JPM Janssen-Mommen. Arch. Environ. Contam. Toxicol., 1993, 25: 3-11.
Martin MH and PJ Coughtrey. Biological monitoring of heavy metal pollution. Applied Science Publication, London, New York, 1982.
Mersch J, A Jeanjean, H Spor and J-C Pihan. In: Limnologie Aktuell (Neumann/Jenner eds.), The Zebra Mussel Dreissena polymorpha, Gustav Fisher Verlag, 1992, p. 227-244.
NAS (National Academy of Sciences, The International Mussel Watch, Washington DC, 1980, pp. 148.
RIZA (Netherlands Ministry of Transport and Public works. Biologische monitoring zoete rijkswateren (in Dutch), Notanummer 96.009, 1996.
Simonich SL and RA Hites. Science, 1995a, 269: 1851-1854.
Simonich SL and RA Hites. Environm. Sci. and Technol., 1995b, 29: 2905-2914.
Thompson TS and RG Treble. Chemosphere, 1995, 31: 4387-4392.