EU legislation to reduce the risk of pharmaceuticals in the environment
Authors:
Lauri Äystö, Finnish Environment Institute, lauri.aysto(at)syke.fi;
Noora Perkola, Finnish Environment Institute, noora.perkola(at)syke.fi
In late October 2022, the European Commission (EC) proposed updates for the urban wastewater treatment directive (EC 2022a) and other water directives (EC 2022b), including the environmental quality standards directive. In both proposals, the level of ambition concerning micropollutants, including active pharmaceutical ingredients (APIs), has increased drastically.
The proposed updates
The directive updates cover everything from wastewaters to surface waters and onto groundwaters, also touching upon greenhouse gas emissions, public health information (e.g. about epidemics) and the circular economy, among other things. The proposed updates are numerous and, in some cases, fundamental.
While the list of monitored parameters in the urban wastewater treatment directive (91/271/EC, UWWTD) previously largely focused on nutrients, the proposal (EC 2022a) requires member states to monitor water framework directive (WFD) priority substances, selected indicator micropollutants, microplastics and antimicrobial resistance, among other things. Moreover, the list of WFD priority substances (EC 2022b) is proposed to be modified, including the addition of 10 APIs as priority substances for surface waters (see Table 1). Additionally, two APIs (carbamazepine and sulfamethoxazole) are proposed as priority substances for groundwaters.
Table 1. The APIs listed in the directive proposals.
|
API
|
CAS
|
EC 2022a
|
EC 2022b
|
UWWTD
|
WFD
EQS
|
Ground-
water QS
|
| 17 alpha-ethinylestradiol (EE2) |
57-63-6 |
|
X |
|
| 17 beta-estradiol (E2) |
50-28-2 |
|
X |
|
| Amisulprid |
71675-85-9 |
X |
|
|
| Azithromycin |
83905-01-5 |
|
X |
|
| Candesartan |
139481-59-7 |
X |
|
|
| Carbamazepine |
298-46-4 |
X |
X |
X |
| Citalopram |
59729-33-8 |
X |
|
|
| Clarithromycin |
81103-11-9 |
X |
X |
|
| Diclofenac |
15307-86-5 |
X |
X |
|
| Erythromycin |
114-07-8 |
|
X |
|
| Estrone (E1) |
53-16-7 |
|
X |
|
| Hydrochlorothiazide |
58-93-5 |
X |
|
|
| Ibuprofen |
15687-27-1 |
|
X |
|
| Irbesartan |
138402-11-6 |
X |
|
|
| Metoprolol |
37350-58-6 |
X |
|
|
| Primidone |
125-33-7 |
|
|
(X)a |
| Sulfamethoxazole |
57-63-6 |
|
|
X |
| Triclosan |
3380-34-5 |
|
X |
|
| Venlafaxine |
93413-69-5 |
X |
|
|
Sum of all detected and
quantified APIs, incl. relevant
metabolites and degradation products |
|
|
|
X |
a) Setting a threshold value to be considered by each member state.
If the proposed updates are accepted, this would be the first time that APIs have received legally binding environmental quality standards in the EU. Furthermore, as priority substances, these APIs would be included in the list of monitored substances in the UWWTD.
Enhanced micropollutant removal
In addition to increased requirements for monitoring, the UWWTD requires quaternary treatment, capable of removing a wide range of micropollutants, to be implemented in a stepwise manner. By 2035 all wastewater treatment plants (WWTPs) with a population equivalent (PE) exceeding 100,000 should have implemented these technologies. Furthermore, WWTPs exceeding 10,000 PE are required to implement similar technologies by 2040 unless the absence of risks to human health and the environment can be demonstrated. This last-mentioned requirement only applies to WWTPs releasing their effluent wastewater to specific types of recipients. While the list of relevant recipients is extensive, it excludes coastal waters that are not used for aquaculture or bathing and where wastewater gets diluted more than tenfold.
Who is going to pay?
The increased costs to WWTPs caused by the monitoring and treatment requirements are proposed to be covered by an extended producer responsibility (EPR) scheme. According to the proposal’s (EC 2022a) background documents, the toxic load reaching the WWTPs mainly originates from pharmaceuticals and personal care products. Thus, the EPR is proposed to only be applied to the pharmaceutical and cosmetic industries.
Has the problem of pharmaceuticals in the environment been solved?
Some parts of the directive proposals would benefit from more concrete definitions. These include, for example, the sum parameter for APIs and their transformation products set for groundwater quality standards, where the maximum allowed limits of quantification and a priority list of the parameters to be included in the analyses should be set. Furthermore, the risk assessments required in the UWWTD needs to be defined with standard procedures. Nevertheless, the proposals are a welcome signal that the work carried out so far in the field of pharmaceuticals in the environment will eventually materialise into EU-level regulation. Moreover, according to EC 2022c, the micropollutant load on the environment is expected to decrease by circa 30% by the year 2040 when implementing the proposed UWWTD. This is a significant fraction, but it still leaves researchers working in the field with some work to do.
References
Creating new incentives for the responsible manufacturing of antibiotic
Author: Radhika Gupta, Project Communications Manager, SIWI, radhika.gupta(at)siwi.org
The Responsible Antibiotics Manufacturing Platform (RAMP) is operating to create new incentives based on criteria that define responsible manufacturing.
The release of antibiotics from manufacturing waste streams into the environment poses global health and business risks. Yet, it remains a challenge to bring about change for two reasons. First, there is a lack of jointly defined objectives and access to verified information that shows progress towards reduction in antimicrobial resistance. Second, there is a lack of enabling conditions that would incentivise and drive demand for improved industry practice.
In an interview with Nicolai Schaaf (‘NS’ in the interview below), Team Lead for Water and Pharmaceuticals, and Iris Panorel (‘IP’ in the interview below), Programme Manager at the Stockholm International Water Institute, we discuss RAMP’s newly developed framework, a work in progress.

Nicolai Schaaf (on the left) and Iris Panorel (on the right). © Photo: Radhika Gupta
What is the role of water governance in reducing the risk of the spread of antimicrobial resistance?
IP: Water is central to reducing the risk of the spread of antimicrobial resistance (AMR). Access to clean water and sanitation is the most basic step in protecting human health from pathogenic diseases and reducing the use of antibiotics. On the other hand, contaminated water sources can act as hotspots and vectors of resistant genes and pathogens.
When it comes to the challenge of AMR, it arises from the lack of collective action by responsible stakeholders. As water plays a central role in addressing this challenge, at SIWI [the Stockholm International Water Institute] we see the need for global water governance and the need to prioritise both the development of policies and their implementation.
NS: The main problem in the space of pharmaceuticals and antibiotics in the environment is that there are no science-based standards. This means that all the engaged stakeholders are pioneering and covering new ground, without a harmonised outcome. Approaching the challenge from a water governance perspective can help to create the right structures.
What is the challenge that RAMP is trying to solve?
IP: Most antibiotics that enter the environment and significantly drive AMR come from usage (by humans and animals) and from the improper disposal of medical waste or unused medicines. RAMP wants to address localised water pollution resulting from the manufacturing of antibiotics, which is a preventable driver. Specifically, it wants to address the exposure of bacteria to selective concentrations that trigger AMR.
Currently, there is a lack of consensus on what responsible antibiotics manufacturing across the value chain should be. RAMP’s goal in this framework is to synthesise and connect the roles and needs of different stakeholders in order to promote policy changes and create enabling conditions for improved industry practice.
More concretely, how is RAMP engaging with the stakeholders?
NS: The character of our work is that we help stakeholders to understand their individual roles and responsibilities, which form part of a larger picture to combat AMR. No one can solve this challenge alone.
There is a critical need to understand that the physical implementation of good practice takes place at the factory level. But in the absence of independent standards, we need to define what needs to be done and how it is going to be implemented, and RAMP is facilitating this dialogue with the industry, solution providers, regulators and scientists.
IP: RAMP has prepared a framework that will take into account the different perspectives of relevant stakeholders from the supply-and-demand side of the antibiotic manufacturing chain. This is an attempt to define and harmonise criteria for responsible antibiotics manufacturing that is scientifically backed and fits the purpose of preventing the risk of AMR.
Our proposal for independent criteria is currently undergoing consultation with scientists, UN agencies, procurers and industry.
What are the key features of the framework?
NS: Inspired by the environmental regulation of the Industry Emissions Directive and the Indian Zero Liquid Discharge policy, the framework recommends building a standard based on targeted interventions and the best available technologies, in addition to the established method of measuring concentrations of APIs [active pharmaceutical ingredients].
The framework finds a balance between the core objective of safe discharge limits and environmental concentrations, and technical achievability and verifiability.
What do you hope to achieve with this framework, and who is it intended for?
IP: The framework is created to help procurers integrate verifiable environmental aspects into their sustainable procurement tools. By taking this concrete requirement into account in their tenders, they can incentivise those companies in the supply chain that are capable of demonstrating good manufacturing practices for antibiotics.
NS: The criteria of the framework will be the connectors between the required physical improvements in manufacturing and how policy and market instruments – including public procurement – can utilise this approach for sustainability criteria.
How will the framework be applicable in the context of Baltic Sea countries?
NS: We want to empower governments and agencies to live up to their responsibility regarding preventing pollution. As the manufacturing of antibiotics is limited in the region, the impact here is rather found in preventing emissions from other parts of the world. We believe that the logic of achieving safe discharge levels and that the means of implementation and verification are applicable to various sources of antibiotics and other pharmaceuticals. This could help in defining guidance for discharge levels or interventions in healthcare facilities, food production and municipal wastewater treatment systems.
About SIWI
SIWI engages with a broad range of water-related topics and has a focus on building stronger societies by improving water governance.
RAMP was launched in 2020. It builds on SIWI’s past work with pharmaceutical industries that target reduction in the spread of AMR.
Researchers from Lithuania and Latvia are obtaining new knowledge on the pharmaceutical pollution of wastewaters and water bodies in both countries
Authors:
Sergej Suzdalev, Marine Research Institute, Klaipeda University, sergej.suzdalev(at)apc.ku.lt;
Michael Stapf, KWB – Berlin Centre of Competence for Water, michaelstapf(at)gmx.net
Although significant efforts have been devoted to the water pollution reduction in both Latvia and Lithuania, and although a substantial decrease in urban pollution has been achieved, a report on pharmaceuticals occurrence in the Baltic Sea that was published in 2017 (UNESCO & HELCOM 2017) indicated major data gaps in Lithuania and Latvia related to sources and pathways, including gaps on the sales and consumption of pharmaceuticals and their concentrations in freshwater and wastewater systems (river water, and influents and effluents of municipal wastewater treatment plants (WWTPs)).

Discharges of effluents from urban wastewater treatment plants are regarded as forming a major source of pharmaceutical pollution in the environment. © Photo: Vanessa Riki
Since February 2021, researchers of the Marine Research Institute of Klaipėda University have cooperated closely with the Latvian Institute of Aquatic Ecology of Daugavpils University and the Latvian Environment, Geology and Meteorology Centre – as well as with other national public and regional institutions – aiming to obtain the lacking knowledge on and experience of pharmaceutical pollution loads and their effects on the environment in order to support the future prioritisation and development of policy measures for the implementation of pharmaceutical pollution mitigation in Latvian and Lithuanian waters (MEDWwater – Pharmaceuticals in wastewaters – levels, impacts and reduction, LLI-527).
Twenty-five pharmaceutical substances investigated in 16 selected places
Based on the agreed criteria, 16 relevant WWTPs and water bodies (eight from Lithuania and eight from Latvia) were selected for further detailed investigations within the MEDWwater initiative. Two sampling campaigns were conducted: in the 2021 summer period (July–August) and winter period (December). Sixty-seven samples of wastewater and 65 samples of water from the selected waterbodies/receivers were collected in both countries during both sampling campaigns and sent to Klaipėda University’s Marine Research Institute for sample preparation and analysis of the 25 selected pharmaceuticals, representing different therapeutic groups.

The WWTPs and waterbodies selected for further investigation within MEDWwater.
The apparent domination of anti-inflammatory and analgesic substances in the wastewaters
The measured concentrations of active pharmaceutical ingredients (APIs) in the WWTP influents varied over several orders of magnitude (ranging from 10 ng/L to 40,000 ng/L) with the highest values typically being for diclofenac, ibuprofen, paracetamol (non-steroidal anti-inflammatory drugs and analgesics), metoprolol (a beta blocker) and oseltamavir (an antiviral medication). A comparison of the median API concentrations in the WWTP influent of both countries showed that most APIs were in a similar range. For five APIs (aciclovir, hydroxychloroquine, ibuprofen, oseltamavir and paracetamol), the median concentrations were more than twice as high in the Lithuanian WWTPs compared with the Latvian WWTPs.
The patterns of the API concentrations in the WWTP effluent were similar to the ones in the WWTP influent, but they had been reduced by about 40–60 %. Only diclofenac and paracetamol were still detected in the WWTP effluent in concentrations above 1,000 ng/L, whereas the majority of the APIs at the Latvian WWTPs had a median concentration of <100 ng/L.
There was a general tendency that the highest concentrations of APIs were detected in the influents and effluents of the largest WWTPs: those at Daugavpils and Liepāja in Latvia and Klaipeda, Šiauliai and Telšiai in Lithuania.
The concentrations of ibuprofen in the receiving water bodies posing an environmental risk
Although no legally binding environmental quality standards (EQSs) are available for the APIs measured in the MEDWwater project, predicted no-effect concentration values from the available scientific studies have been used to calculate risk quotients based on the average API concentrations in water samples taken downstream of the investigated WWTPs. In the receiving water bodies, only the biodegradable ibuprofen had concentrations above its proposed EQS (10 ng/L) at the sampling point and, thus, could cause an environmental risk if the annual average concentration was at this level.
The readiness of Lithuanian and Latvian WWTPs to implement advanced treatment technologies
In order to fill the knowledge gap and get examples of WWTPs optimisation options – seeking to improve the removal rates of pharmaceuticals during the treatment process – a foreign expert (Dipl.-Ing. Michael Stapf) from Kompetenzzentrum Wasser Berlin (KWB) has been outsourced to deliver technical consultation for the selected WWTPs. Based on the available data for the 16 selected WWTPs in Latvia and Lithuania, it was concluded that most of the investigated WWTPs are not suitable for a powdered activated carbon treatment because of the manner of their current excess sludge disposal. Due to the lack of information on bromide and nitrite concentrations, it is currently not possible to assess the suitability of ozonation. There are no obvious barriers to the implementation of a granulated activated carbon filtration. At the moment, most of the investigated WWTPs do not have an existing tertiary treatment stage that can be used in combination with API elimination technologies (e.g. ozonation post-treatment).

(This publication has been produced with the financial assistance of the European Union. The contents of this publication are the sole responsibility of Project Partners and can under no circumstances be regarded as reflecting the position of the European Union.)
The Finnish environmental classification of medicines offers information about the risks medicines pose to aquatic environments
Author: Elli Leppä, Development Pharmacist (PhD), Pharmaca Health Intelligence, elli.leppa(at)pharmaca.fi
Health care professionals, like doctors and pharmacists, face increasing numbers of patient and customer questions concerning the environmental effects of medicines. In Sweden the FASS environmental classification has been used for over 15 years to convey information about the risks of medicine residues in aquatic bodies. In Finland there was no similar classification until December 2021, when Pharmaca Health Intelligence published the Finnish environmental classification for health care professionals.

Medicine residues can have a negative impact on aquatic environments. © Photo: Elli Leppä
Most consumers and many health care professionals are unaware that appropriately used medicines currently have the heaviest impact on the environment. A common misconception is that, if unused, medicines are disposed of responsibly and the environmental impact of medicine use approaches zero. To reduce the environmental load from medicines, more attention should already be paid when choosing treatments in addition to paying attention to the disposal phase of the medication process. To reduce the effects on, for example, aquatic environments, the abundant research data concerning medicines in the environment has to be formulated into clear, user-friendly tools in order to help health care professionals in choosing the least environmentally harmful medicines in situations when such choices are possible to make.
Rational drug therapy should focus on the patient’s needs and promote the use of the necessary medicines. Care should be taken that medicine information does not cause any undue concern for the medicine’s users (e.g. regarding the environmental safety of the medicine). However, in situations where several therapeutically equivalent options are available to treat the patient’s disease or symptoms, clear and concise information about the environmental effects of medicines may be one of the selection criteria that health care professionals can use to choose the most suitable option.
In a small study about the needs of pharmacy professionals for environmental information about medicines (Minkkinen et al., 2020), 68% of the respondents (pharmacists currently working in pharmacies) reported that finding information about the environmental effects of medicines was difficult or very difficult. The great majority (83%) of the respondents thought that a Finnish environmental classification should be created to support the information needs of health care professionals. In December 2021 Pharmaca Health Intelligence (previously the Pharmaceutical Information Centre) published the Finnish environmental classification of medicines as a part of the Pharmaca Fennica compendium of medicine information for health care professionals. The classification is based on the Swedish FASS classification (www.fass.se) and includes information about the risk class, bioaccumulation and degradation profiles of medicines.
The environmental classification is substance based and covers medicines for human use. The risk class is presented both as text and in symbol format. The risk is divided into five classes, with additional degradation and bioaccumulation information. The risk level is influenced by both the toxicity (implied by the predicted no-effect concentration (PNEC)) of the medicine and the extent of exposure (the predicted environmental concentration (PEC)). The total sales of the substances in Finland are used to calculate the amount of exposure. Substance groups which are known to have little to no environmental effects (e.g. vitamins, proteins) have been placed in the lowest (‘no effect’) class.
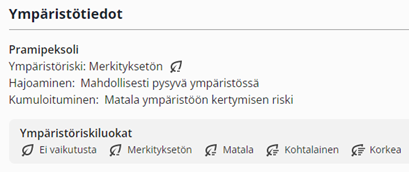
The environmental information for pramipexole, a medicine used for Parkinson’s disease.
The first phase of the classification is currently available to health care professionals via the Pharmaca Fennica Premium online service. Development of the classification continues. The classification is eventually expected to expand to veterinary medicines and the life cycle effects of medicines (e.g. carbon footprint evaluation results) when research data on those subjects becomes more widely available. Other development themes under consideration are the wastewater clearance of substances and how to make the EU watchlist substances more prominent in the classification.
Further reading:
Do you have something you would like to share to the BSR PHARMA?
Tips on contents for future newsletters are welcomed at BalticPharma(at)syke.fi