New report on micropollutants in BSR wastewater treatment plants
Authors:
Lauri Äystö, Finnish Environment Institute, lauri.aysto(at)syke.fi
Dmitry Frank-Kamenetsky, HELCOM secretariat, dmitry.frank-kamenetsky(at)helcom.fi
In 2017, the UNESCO & HELCOM status report on pharmaceuticals in the aquatic environment of the Baltic Sea region revealed the presence of several active pharmaceutical ingredients (APIs). A new report, recently approved for publication in the BSEP Series, provides further insight into the problem of contamination of the BSR environment, particularly, emissions of a wide range of micropollutants, including over 100 pharmaceuticals, at wastewater treatment plants within the region.

Wastewater treatment in Finland. © Photo: Lauri Äystö.
The most recent HELCOM holistic assessment (HOLAS II) found all parts of the Baltic Sea to be under pressure from contaminant emissions. However, for a long time, HELCOM work has mainly focused on a handful of indicators originating from the list of priority contaminants established more than a decade ago, such as polybrominated diphenyl ethers, polyaromatic hydrocarbons and polychlorinated biphenyls. As a result, several groups of so called micropollutants have previously been largely overlooked in HELCOM work.
In 2015, HELCOM launched a data call on active pharmaceutical ingredients (APIs) in the Baltic Sea environment. This data call resulted in the UNESCO & HELCOM status report, highlighting the widescale presence of these substances in the aquatic environment of the Baltic Sea catchment area. However, following the conclusions of the report, and implementing a HELCOM joint action on micropollutants in wastewater treatment plant (WWTP) effluents, a further data call was launched in 2017. Its aim was to produce a more comprehensive picture of micropollutant emissions within the Baltic Sea region. The data call covered several classes of micropollutants, such as alkylphenols, perfluoroalkylsubstances, heavy metals and pharmaceuticals.
The reported data was processed in a collaborative effort between the HELCOM secretariat and projects BSR-water, CWPharma and BONUS CLEANWATER. For pharmaceuticals, the data produced in the 2015 and 2017 data calls were merged to create the most comprehensive data compilation on API occurrence in WWTPs within the Baltic Sea region. The resulting report was approved for publication in the Baltic Environment Proceedings (HELCOM HOD 61-2021) and will be published early 2022.
Data coverage
The work on pharmaceuticals focused on API emissions from wastewater treatment plants. The dataset on pharmaceuticals consisted of circa 10,000 datapoints, covering over 100 WWTPs and 117 individual substances. While the dataset had a relatively good geographical coverage of seven BSR countries, the countries were not represented uniformly. Data reported from Denmark accounted for over 60% of all datapoints, while Germany and Finland accounted for 16% and 14%, respectively. The remaining 10% of datapoints were reported from Sweden, Estonia, Latvia and Poland. Similarly, sewage sludge as a sampling matrix was underrepresented in the dataset. Sludge samples accounted for only 8% of all samples, while influent and effluent samples accounted for 33% and 59%, respectively.
Pharmaceutical emissions from wastewater treatment plants
The APIs present in the highest median concentrations in effluent wastewater included, among others, the anticonvulsant gabapentin (med 6,800 ng/L, n=17), the contrast agent amidotrizoate (med 4,900 ng/L, n=252), and the diuretic furosemide (med 3,600 ng/L, n=247). Out of these three APIs, only furosemide was also analysed in influent samples. Its concentrations in influent water samples were within the same order of magnitude as in effluents (med 7,200 ng/L, n=184).
While the concentrations of the APIs considered in the EU Water framework directive (2000/60/EC) watchlists were not the ones detected in the highest effluent concentrations, they often exceeded their proposed environmental quality standards (EQS). For instance, 93% of the quantified diclofenac concentrations (med 2,200 ng/L, n=249) in effluent samples exceeded the EQS proposed for the substance. Similarly, azithromycin (med 90 ng/L, n=11) and erythromycin (med 13 ng/L, n=71) exceeded their proposed EQS-values in 91% and 41%, respectively. Also, the hormones 17β-estradiol, 17α-ethinylestradiol and estrone exceeded respective EQSs in a vast majority of quantified samples, sometimes several orders of magnitude. It is also noteworthy, that for these substances the limits of quantification were often higher than the proposed EQS-values.
Way forward
The report leaves no doubt as to whether APIs are present at WWTPs and discharged into the environment. Moreover, the report supports previous observations that there are several APIs that are not removed efficiently in conventional WWTPs.
While the report confirms that APIs are continually leaking into the Baltic Sea environment, the data on API occurrence is still very fragmented. To identify the APIs posing the highest risks to recipient environments, as the first step, comprehensive dataset consisting of a representative number of observations and applying analytical methods of sufficient precision would be needed. This dataset, in combination with relevant ecotoxicological data, could then be utilized to prioritize APIs for establishing regional and national monitoring programmes and to develop measures to reduce their input to the aquatic environment.
_______________________________________________
Pharmaceuticals to be included for the first time in WFD priority substance list?
Author: Jukka Mehtonen, Finnish Environment Institute, jukka.mehtonen(at)syke.fi
The revision of the list of WFD priority substances is going on now and pharmaceuticals are well represented among candidate substances. The EQS dossiers are now being prepared but it takes several years until new priority substances are entered into force at a national level. EU wide water related Watch lists are big steps forward in the risk assessment of contaminants in Europe.
Background
The Water Framework Directive (WFD) was established to protect inland surface waters, coastal waters and groundwaters. The WFD requires achieving a good chemical status for surface water and groundwater. A good chemical status is achieved for a surface water body when all environmental quality standards for the priority substances and other pollutants listed in Directive 2013/39/EU, the so-called ‘Daughter’ Directive of the WFD, are complied with. It defines a European priority list of substances posing a threat to or via the aquatic environment with respective environmental quality standards (EQS). EQS in the WFD define the concentration of a substance in water, sediment or biota, which is regarded as safe for the environment and human health and which must, therefore, not be exceeded. Currently, in 2021, Directive 2013/39/EU lists 45 substances or substance groups to WFD Annex X (Annex of EU priority substances).
The European Commission reviews the list of priority substances every 6 years, according to Art. 1 2013/39/EU. In practice, the list has only been reviewed twice: in 2008 (2008/105/EC) and in 2013 (Directive 2013/39/EU) since the setting of the priority substance list for first time in 2001. Art. 16 of WFD introduces a scientifically based methodology for selecting priority substances based on their significant risk to or via the aquatic environment.
The Fitness Check published in December 2019 concluded that WFD is broadly fit for purpose, but a problem related to a slow process of the revision of the list of WFD priority substances is evident and must be solved.
Pharmaceuticals – status in the WFD prioritization process
The working group dealing with priority substances under the WFD Common Implementation Strategy (CIS) is the Working Group Chemicals (WGCHEM). The substances have been shortlisted mainly by JRC and then selected by the WGCHEM based on monitoring-supported exceeding of the PNEC value and modelled data. There is evidence that these substances may pose a significant risk to the aquatic environment or human health. The selection of the substances is being and has been followed by a publicly open and transparent discussion with interested parties.
The revision of the list of WFD priority substances started in 2015, being interrupted during 2018-19 due to the WFD Fitness Check, but continued in 2020. The revision work includes not only the listing of new priority substances, but also considers the revision of EQS values and de-listing of current priority substances (de-listing not previously done).
So far (November 2021) the EQS values are being drafted for most shortlisted substances (substance specific EQS dossiers) which include several pharmaceuticals. Substance specific working groups containing JRC, Member State experts, industry and other interested stakeholders draft the EQS dossiers. The pharmaceuticals are well represented among candidate substances. It may indicate that pharmaceuticals will be included in the WFD priority substance list for the first time.
The short-listed pharmaceuticals are three macrolide antibiotics (erythromycin, clarithromycin & azithromycin), carbamazepine, diclofenac, three hormones (estradiol, ethinylestradiol and estrone) and ibuprofen. At the moment, for almost all the candidate pharmaceuticals, the EQS draft dossiers have been prepared and sent to the Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) in order to get its scientific opinion on dossiers. Nevertheless, for some substances such as ibuprofen the preparation of EQS dossiers has just started. Thus, EQS dossiers are at a very different preparation stage.
European Union Strategic Approach to Pharmaceuticals in the Environment published in March 2019 by the Commission gives support for listing some pharmaceuticals in the WFD priority substance list.
Watch list mechanism
This WFD priority substance revision is the first time when the results of the surface water Watch List mechanism will be utilized. To support the monitoring-based process for the review of the list of priority substances, a watch list has been drawn up which is published every two years and contains around 10 substances suspected of exceeding EQS across Europe. The substances on the watch list are measured by all Member States and, if the proposed EQS is actually exceeded, may become candidates for the priority substances list (Art 8b WFD “daughter directive” 2013/39/EC). The main goal of the Watch List mechanism is to obtain high quality and comparable environmental monitoring data from all Member States. The Watch List only concerns authorities. Thus, regarding substances listed on the watch list, there is no direct obligation to undertake specific water management measures. The first Watch List was established in 2015 (2015/495/EU) and updated in 2018 (2018/840/EU) and 2020 (2020/1161/EU). A relatively high number of pharmaceuticals have been listed in all three surface water Watch Lists. The preparation of the fourth surface water Watch List is to be started in 2022.
The first groundwater Watch List, based on Groundwater Directive (2006/118/EC), is currently being prepared, but it will be voluntary for Member States and the substances have not yet been defined. The new Drinking Water Directive (2020/2184/EU) includes, for the first time, the Watch List substances for water intended for human consumption. The Watch List includes two substances, one of them being estradiol.
EU wide water related Watch Lists are big steps forward in the risk assessment of contaminants in Europe. Nevertheless, some harmonization is needed regarding the type of sampling points; i.e. if they are heavily or semi-polluted, or like background areas.
Schedule for further work
SCHEER has already given feedback for some candidate substances and is still preparing its scientific opinion on some draft EQS dossiers. This may take place at the beginning of 2022. The substance specific working groups will then further refine the EQS dossiers based on SCHEER feedback. The Impact Assessment of the Follow-Up to the WFD Fitness Check with a connection to a revision of the list of WFD priority substances has been scheduled to be finalized by June 2022. The European Commission may submit, perhaps at around the end of 2022, a proposal on the list of WFD priority substances with respective EQS values. After negotiations with Member States, industry and other stakeholders, the adoption of the list of WFD priority substances may take place at the end of 2023. The final decision to amend the list is to be taken with the approval of a so-called daughter directive based on the common legislative procedures. The Member States shall bring into force the laws, regulations and administrative provisions necessary to comply with the Directive amended WFD priority substance list within around two years after adoption (around 2025/ 2026).

_______________________________________________
The updated Baltic Sea Action Plan – towards a Baltic Sea unaffected by hazardous substances
Authors:
Susanna Kaasinen, HELCOM Secretariat, susanna.kaasinen(at)helcom.fi
Owen Rowe, HELCOM Secretariat, owen.rowe(at)helcom.fi
The HELCOM Ministerial Meeting held on 20 October 2021 adopted the updated HELCOM Baltic Sea Action Plan (BSAP) containing almost 200 actions to improve the status of the sea. The updated plan has a segment dedicated to hazardous substances and litter with the goal “Baltic Sea unaffected by hazardous substances and litter”.
The Baltic Sea Action Plan (BSAP) is HELCOM’s strategic programme of measures and actions for achieving good environmental status of the sea, ultimately aiming for a Baltic Sea in a healthy state. Initially adopted by the HELCOM Contracting Parties – the nine Baltic Sea countries plus the European Union – in 2007, the original BSAP had set 2021 as the target year for achieving good ecological status of the sea. However, the results of the State of the Baltic Sea report, covering the years from 2011 to 2016, already indicated in 2018 that this target would not be met.
The updated BSAP is based on the original BSAP and maintains the same level of ambition. It also retains all actions previously agreed on that are still to be implemented, while it additionally includes new actions to strengthen the existing efforts and tackle emerging concerns.
Guided by the HELCOM vision of “a healthy Baltic Sea environment with diverse biological components functioning in balance, resulting in a good ecological status and supporting a wide range of sustainable economic and social activities”, the updated BSAP is divided into four segments with specific goals:
- Biodiversity, with its goal of a “Baltic Sea ecosystem is healthy and resilient”,
- Eutrophication, with its goal of a “Baltic Sea unaffected by eutrophication”,
- Hazardous substances and litter, with its goal of a “Baltic Sea unaffected by hazardous substances and litter”, and
- Sea-based activities, with its goal of “Environmentally sustainable sea-based activities”
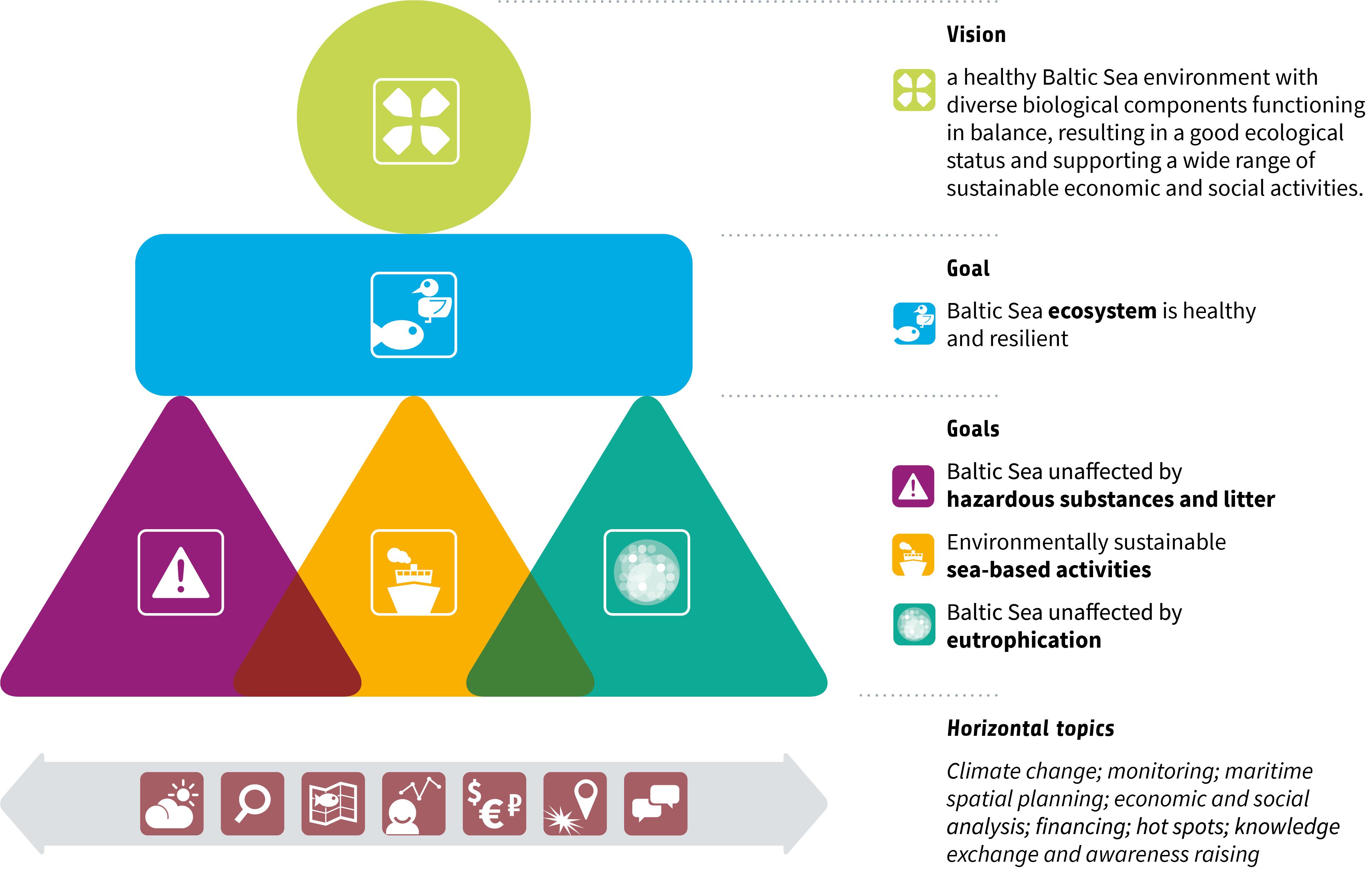
Each of the four segments is structured around the updated HELCOM ecological and management objectives and contains concrete measures and actions to be implemented by 2030 at the latest.
Hazardous substances in the updated BSAP
The majority of the actions related to hazardous substances are included in the hazardous substances and litter segment of the BSAP. The ecological objectives regarding hazardous substances are:
- Marine life is healthy;
- Concentrations of hazardous substances are close to natural levels;
- All sea food is safe to eat;
- Minimal risk to humans and the environment from radioactivity.
And the management objective is:
- Minimize input and impact of hazardous substances from human activities.
The segment includes 30 actions on hazardous substances with the aim of addressing the wide array of hazardous substances and human activities involved in their release to the environment. One of the key actions is to develop a regional strategic approach and, on the basis of that approach, an action plan for HELCOM work on hazardous substances by 2024 (action HL1). This holistic approach is needed to address not only the legacy pollutants such as heavy metals, dioxins or organotins, but also contaminants of emerging concern such as per- and polyfluoroalkyl substances (PFAS) and pharmaceuticals.
Furthermore, specific actions require improved awareness related to the appropriate disposal and handling of hazardous substances (e.g. actions HL7 and HL25), or improved consumer knowledge of the impact of pharmaceuticals in the environment (action HL27). Moreover, several of the actions are also directly targeting contaminants of emerging concern, for example, by taking concrete measures, such as strengthening the collection of unused pharmaceuticals from the public (action HL26), by filling knowledge gaps, such as to improve the knowledge related to the occurrence of pharmaceutical substances in the environment, their persistence and harmful effects (action HL22), or by evaluating priority pharmaceutical substances and developing guidance for monitoring, analysis and potential indicator development (actions HL23 and HL24).
______________________________________________
SUDDEN – tackling the environmental impacts of medicines across their life cycle
Authors:
Jari Yli-Kauhaluoma, Tiina Sikanen & Sanja Karlsson, University of Helsinki
Kari Jalonen, Demos Helsinki
Correspondence to: Project coordinator, sanja.karlsson(at)helsinki.fi
SUDDEN is a multidisciplinary research program that aims to reduce environmental impacts of medicines across their life cycle from development until use, in close cooperation with experts in the pharmaceutical industry, healthcare, and policymaking.

Finnish lakeside. © Photo: Sanja Karlsson.
Background
Medicines are vital for human wellbeing and public health, but their increased consumption - resulting from population growth and ageing - inevitably increases the pharmaceutical residues in the environment, as well as the medicine waste across the drugs’ life cycles. SUDDEN project, short for Sustainable Drug Discovery and Development with End-of-life Yield, seeks for new solutions to the management of environmental hazards of medicines at the molecular and product levels, and to environmentally sound policies that could support the rational use of medicines and sustainability of the ancillary healthcare systems. The SUDDEN research program (2018-2023) receives funding from the Strategic Research Council (SRC) established within the Academy of Finland and is part of SRC’s Keys to Sustainable Growth programme.
The SUDDEN consortium
The SUDDEN consortium is coordinated by the University of Helsinki and involves five other Finland-based research organizations, uniting experts in drug discovery and pharmacoeconomics, international environmental law and risk assessment, water purification technology, metal and plastic recycling, and transformation research: University of Helsinki, Aalto University, LUT University, Finnish Environment Institute SYKE, University of Eastern Finland and the Demos Helsinki.
Elimination of pharmaceutical residues in wastewaters and sewage sludge
The majority of pharmaceutical residues pass the municipal wastewater treatment processes unchanged, distributing to the receiving water bodies and/or the sewage sludge. For one part, the focus on ongoing SUDDEN research is to evaluate and improve the purification efficiencies of different tertiary technologies, such as adsorption to activated carbon, oxidation via pulsed corona discharge and membrane filtration, in the removal of pharmaceutical residues from wastewaters (LUT University). For the other part, the ongoing research focuses on safe recycling of sewage sludge by building a better understanding of the fate of pharmaceutical residues in the different sludge treatment processes, such as digestion and composting, pyrolysis, and incineration, as well as in sludge-fertilized soil (Finnish Environment Institute SYKE).
Safe and efficient recycling of pharmaceutical packaging waste
Packaging of pharmaceutical products aims at ensuring the high quality and safety of medicines to the user, including protection against falsified medicines. In some cases, however, this necessitates the use of environmentally damaging materials (e.g., PVC) or rare metals (especially aluminum), but there are also regional preferences that augment the use of certain problematic packaging types, such as the favoring of PVC-aluminum blisters as primary packages in the EU. To manage this regionally relevant waste segment more effectively, SUDDEN research focuses on the development of metallurgy methods for the processing of blister waste (Aalto University) in a way that could facilitate sustainable aluminum recycling, instead of its incineration (current norm). Adhering to the EU’s plastics strategy, SUDDEN also evaluates safe recycling practices for plastics used in pharmaceutical primary packages (Finnish Environment Institute SYKE).
Research highlights: Electrohydraulic Fragmentation of Aluminum and Polymer Fractions from Waste Pharmaceutical Blisters (ACS Sust. Chem. Eng. 2020).

Recycling pharmaceutical packaging materials, such as blisters, is looked into in the project SUDDEN. © Photo: SUDDEN.
New strategies for pharmaceuticals environmental risk assessment
Given the fact that the current pharmaceutical legislation, or the regulatory framework governing environmental risk assessment (ERA), are forceless in limiting the environmental exposure resulting from the human use of medicines, it is utmost critical to understand the validity of regulatory ERA data in predicting the true environmental hazards. In this respect, SUDDEN research focuses on exploring the prevailing methodological limitations and identifying the knowledge gaps in the regulatory ERA (Finnish Environment Institute SYKE and University of Helsinki). This is further supported by regulatory analysis of the feasibility of the “(benign) by design” approach to support progressive risk governance of innovative technological developments and to enhance the interdisciplinarity in risk regulation (University of Eastern Finland, Law School). SUDDEN also aims to introduce and validate a range of in vitro effects models for fish – the species most sensitive to nontarget effects of environmental drug residues - that could foster the use of nonanimal assays for ERA purposes (University of Helsinki). These activities address the prevailing ethical gap in regulatory ERA, and also interlink SUDDEN with the ongoing PREMIER project (2020-2026), funded under the Innovative Medicines Initiative, by the EU and the European Federation for Pharmaceutical Industries and Associations.
Research highlights:
“By Design” and Risk Regulation: Insights from Nanotechnologies (Eur. J. Risk Regulation 2020).
Cytochrome P450 Inhibition by Antimicrobials and Their Mixtures in Rainbow Trout Liver Microsomes In Vitro (Environ. Toxicol. Chem. 2021).
Environmental and financial sustainability of the healthcare systems and pharmaceutical industry
Besides regulatory requirements, environmentally sound decision-making needs to extend from individuals to societal systems, and, eventually, to the development of pharmaceuticals intrinsically less harmful for the environment. To account for the impact of cultural variation, a focus point of SUDDEN research is to understand the consumers’ attitudes toward and willingness to pay for environmentally sound medicines (University of Eastern Finland, School of Pharmacy). Together with the Swedish Knowledge Center, SUDDEN has also explored national approaches to promote the adaptation of environmental criteria in the public procurement of medicines, supported by evaluation of national and international regulation applied to drug production and distribution chains (University of Eastern Finland, Law School). Furthermore, SUDDEN aims to support the development of greener drug manufacturing schemes and to incorporate the environmental fate (predictions) as part of the computational drug discovery and chemoinformatics workflows (University of Helsinki), in a way that does not create significant cost barriers to the overall (industrial) drug development process. Altogether, these activities focus on the identification of tangible incentives for pharmaceutical industry and policymaking, with the overall aim of reducing the environmental impact of medicines across their life cycle.
Research highlights: Attitudes and Considerations towards Pharmaceuticals-Related Environmental Issues among Finnish Population (Sustainability 2021).
Moving forward
To accelerate the translation of research results to product and service innovations, SUDDEN is about to kick-off an Innovation Brokerage Program in 2022, which seeks to connect the project researchers with practitioners in chosen sectors in the effort of facilitating further development and implementation of research-based innovations as part of environmentally sound decision-making on all levels. More information on the SUDDEN research findings, as well as of past and upcoming stakeholder activities, is communicated via the project website.
_______________________________________________
Do you have something you would like to share to the BSR PHARMA? Tips on contents for future newsletters are welcomed at BalticPharma(at)syke.fi.
___________________________________________